A detailed look at how the US Farm Bill of 2014 & 2018, the DEA, and FDA play a role in the development of Canna-Pet and the legal status of hemp-based products in America.
Canna-Pet® is truly unique. As the pioneer of hemp-based CBD for pets, we’ve had the unique opportunity to pioneer the hemp industry since 2013. A plant-based, benign product made with full spectrum hemp — formally registered as cannabinoid nutrition adapted for pets with the United States Patent and Trademark Office.
Since the beginning, our goal has been to use American grown hemp in our America-made products, to improve the quality of life and nutritional health of every animal on earth. To reach those goals, Canna-Pet® has operated by three basic principles:
First, our products derive from the hemp plant and are made in cGMP laboratories and are certified benign and safe for all animals;
Second, our products work and we guarantee satisfaction
Third, we follow DEA, FDA, and local guides to ensure our products are legal and available without a prescription.
“CBD works in a variety of ways and, surprisingly, it has no side effects. As a matter of fact, it is completely non-toxic.”
Dr. Raphael Mechoulam, Organic Chemist and Professor of Medicinal Chemistry, Hebrew University, Jerusalem
<<discovered THC, isolated CBD in 1963, “Father of the endocannabinoid system”>>
Our Journey to Hemp Commercialization:
2013
Canna-Pet® created the “CBD for pets” industry with our full spectrum, cannabinoid-rich, industrial hemp products in 2013. At that time we had to import all hemp from Europe, because industrial hemp could not be legally grown in the USA at all. Our innovative products remain unique, and our formulations protected.
2014
The 2014 version of the US Farm Bill allowed Canna-Pet® to begin working with American farmers, research universities, and hemp crops. Hemp legalization allowed us to start relationships that now financially benefit thousands of Americans who contribute to, and are impacted by, the hemp business — starting with hemp farmers and product manufacturers — extending to ultimately benefit small towns, as industrial hemp proceeded from research to becoming an agricultural commodity as a commercial crop.
In September of 2014, Canna-Pet® pioneered the domestic USA hemp industry by producing the first commercial products made with American-Grown hemp in over 60 years.
2015
After almost two years of deliberations, the US Patent and Trademark Office awarded official registration to Canna-Pet® — formal recognition that Canna-Pet® is cannabinoid nutrition adapted for pets. Canna-Pet’s formal registration is unique – there are no other cannabinoid nutritional products recognized by USPTO.
Following our groundbreaking USPTO registration in 2015, the FDA objected to language on our website, including comments made in customer testimonials regarding the healing capabilities of hemp and CBD products. Similar warning letters were issued to Diamond Walnuts, and POM Juice for health-related claims. We immediately complied with all language changes requested by the FDA, and we have had no issues subsequent to correcting the website in 2015.
“We are very excited to have partnered with Canna-Pet® and Pet Conscious to explore the potential of cannabinoid-based products to improve the health and well being of dogs suffering from anxiety, chronic lameness, cancer, and epilepsy.”
Dawn M. Boothe, DVM, MS, PhD,
Director, Clinical Pharmacology Laboratory
Auburn University College of Veterinary Medicine
2016-2017
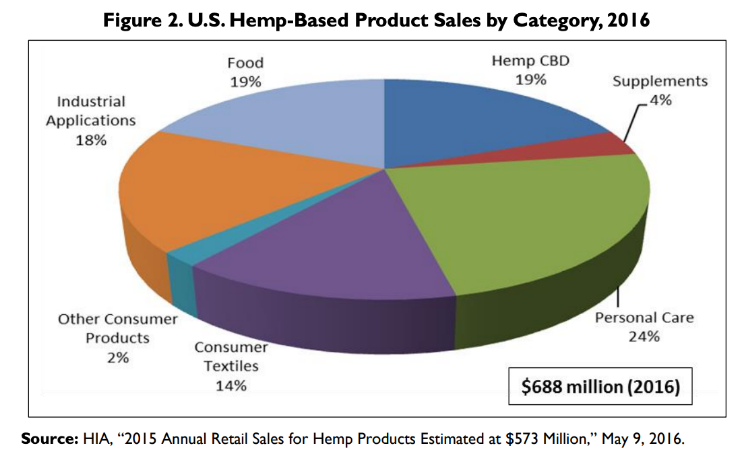
Canna-Pet® celebrates three years of production using exclusively US grown hemp. Hemp CBD is 1/5th of the American market for hemp products – and rapidly growing.
Canna-Pet® was featured in The New York Times “Pets on Pot” which brought national attention to the confusing status of hemp and CBD for dogs, cats, and other pets. Canna-Pet® is covered in Newsweek, CNBC, Rolling Stone, Popular Science, USA Today, People Magazine, Town & Country, petMD, the Journal of the American Veterinary Medical Association, and more – as hemp awareness has grown internationally.
However, with these industry wins came new struggles. The US Department of Agriculture offered to register Canna-Pet®’s hemp product varieties as pharmaceutical controlled substances (as part of clinical research applications with universities) – which we refused and continue to refuse in today’s changing market. The DEA also caused Auburn University clinical research involving our products to be delayed, denied them approvals for their clinical work, and put our research on cancer, seizure, and pain in dogs, cats, and horses on hold for over two years.
In September of 2017, CBD is overtly permitted by the World Anti Doping Agency (WADA) – a global organization which oversees the Olympics, FIFA, international professional and amateur sports.
2018
In 2018, Canna-Pet® celebrated 5 years as the global leader in cannabinoid nutrition, with customers in over 50 countries. For many years Canna-Pet® has worked to educate veterinarians, lawmakers, and the public in the USA about industrial hemp and cannabinoid science. Our efforts were rewarded with the formal protection of industrial hemp as an American agricultural/food crop with the 2018 Farm Bill’s passage into law. In December of 2018, the Farm Bill was formally signed into law and hemp prohibition officially ends!
One day after the 2018 Farm Bill was signed the FDA issued further guidance on hemp – their “pathway for commercialization” officially declared several forms of hemp – including hulled hemp seed, hemp seed protein, and other forms used in Canna-Pet® products – as GRAS (Generally Recognized As Safe) and therefore universally permitted in food and supplements.
Canna-Pet® attained significant progress in the advancement of hemp-CBD clinical research in animals, when the DEA acquiesced and allowed Canna-Pet® CBD-rich products to proceed in Auburn’s research trials as proprietary, full-spectrum hemp, without designation as a pharmaceutical or controlled substance.
However, the end of the prohibition on hemp has not necessarily ended the purported prohibition on discussion of “CBD” in the context of nutrition, as the FDA still hopes to make CBD a pharmaceutical/drug when extracted from marijuana and purified as an isolate — such as in Epidolex®. The FDA still specifically precluded discussion or mention of therapeutic applications of CBD in products while pharmaceutical applications (drugs) with the compound are still under review/consideration.
Thankfully, the FDA for some time has clearly carved out pharmaceutical isolates — compounds like “CBD” or “CBD and THC”— separately from full spectrum industrial hemp products like Canna-Pet®. In June of 2018, the World Health Organization (WHO) confirms that CBD has no abuse or dependence potential in people or animals. Two separate reports from the WHO found that CBD is: safe, non-toxic, non-addictive, non-psychoactive, and has significant potential as a health aid.
About “cannabinoids” and “CBD” with regards to Canna-Pet®
If we attributed our guaranteed results to any particular substance, compound, or cannabinoid, or if we provided testing that explicitly promoted particular compounds:
- We could be precluded from selling our products over the counter by the FDA
- We could be forced to label Canna-Pet® as a pharmaceutical
- We could be forced to stop using hemp from US farmers
We continue to provide our American-grown, full spectrum, proprietary, USPTO registered products over the counter and direct to consumer, with an absolute satisfaction guarantee – as we have done since 2013.
To date, our policies have served hundreds of thousands of customers, and our products have helped millions of pets worldwide – as you can see from customer testimonials and independently moderated reviews on every single product page.
Since 2013 Canna-Pet® has spoken, written and explained in great detail about cannabinoids and about the emerging industrial hemp industry to the DEA, FDA, the USPTO, to veterinary associations, universities, the media, and the public.
To date, our PetConscious Foundation has sponsored more cannabinoid research for animals than any organization on earth, and we have a comprehensive slate of clinical trials with Canna-Pet® products underway with Auburn University through 2022.
2020 & Beyond
Today Canna-Pet® products represent the pinnacle of plant-based wellness for pets, helping millions of pets globally. We create our 100% American-made, organic products exclusively with American-grown hemp.
As one of the largest manufacturers of hemp CBD products in the world, our hemp is sourced from many states – presently it is grown in Colorado, Kentucky, Oregon, and North Carolina – and we are thrilled to already be speaking with farmers in 27 other states that are now moving into hemp production!
Exclusive hemp & CBD research is underway with Canna-Pet® products at Auburn University College of Veterinary Medicine.
We encourage you to try our products for your pet, and see the results in your own home – that is why all our products come with a complete satisfaction guarantee.
Follow our story in The New York Times “CBD Mogul Revives an Architectural Gem in Los Angeles“
Canna-Pet® IS:
- 100% organic plant
- Over the counter, available worldwide
- Benign & Safe
- American-grown hemp
- UNIQUE & GUARANTEED
Canna-Pet® IS NOT:
- Derived or synthetic
- A pharmaceutical, drug, or controlled substance
- A cannabinoid isolate
- A “marijuana”-derived isolate like Epidiolex®
- Like any “CBD product”
You will come to understand why our customers don’t just try or ‘like’ Canna-Pet®. Our customers LOVE Canna-Pet®.
Canna-Pet® is 100% Plant, 100% Organic, 100% American-grown, 100% American-made.
Canna-Pet® guarantees the safety and efficacy of our products.
Sources Used:
Burns, Janet. “WHO Report Finds No Public Health Risks Or Abuse Potential For CBD.” Forbes, Forbes Magazine, 30 Apr. 2018, www.forbes.com/sites/janetwburns/2018/03/18/who-report-finds-no-public-health-risks-abuse-potential-for-cbd/#47a640723476.
Harel, Monica Corcoran. “CBD Mogul Revives an Architectural Gem in Los Angeles.” The New York Times, The New York Times, 23 May 2019, www.nytimes.com/2019/05/22/style/sowden-house-lloyd-wright.html.
Holson, Laura M. “Pets on Pot: The Newest Customer Base for Medical Marijuana.” The New York Times, The New York Times, 8 Oct. 2016, www.nytimes.com/2016/10/09/fashion/pets-medical-marijuana-dogs-cats.html.
Lewis, Amanda Chicago. “Mitch McConnell: Drug Warrior, CBD Champion?” Rolling Stone, 1 July 2018, www.rollingstone.com/politics/politics-features/mitch-mcconnell-drug-warrior-cbd-champion-667089/.
“Mechoulam: The Father of Modern Cannabis Medicine.” Royal Queen Seeds, www.royalqueenseeds.com/blog-mechoulam-the-father-of-modern-cannabis-medicine-n516.
“SUMMARY OF MAJOR MODIFICATIONS AND EXPLANATORY NOTES.” World Anti-Doping Agency, https://www.wada-ama.org/sites/default/files/prohibited_list_2018_summary_of_modifications_en.pdf